Defeating Resistance
In western Kenya in 1990, David Fidock, PhD, first witnessed the raw impact of malaria. He had traveled there to study the human immune response to malaria parasites, but what he saw in the clinics was devastating. “You could see kids coming in who were already in a coma, and you knew that most of them wouldn’t make it to the next day,” he recalls. “It was shocking and humbling to understand that for these people, malaria was still an immutable part of life.”
Even beyond witnessing the suffering malaria caused on an individual level, Dr. Fidock, now a professor of microbiology & immunology and of medical sciences (in medicine) at P&S, saw it in broader terms. “Malaria suppresses developing countries—in Africa in particular—and keeps them from being able to fully develop economically and politically.”
The trip solidified his personal commitment to malaria research. “It led me to adopt an increasingly translational research agenda that focuses on understanding how antimalarials act, what causes treatment failure, and using that information to develop new generations of antimalarial drugs.”
While malaria was eliminated in the United States by 1951, efforts to eradicate it from the rest of the world foundered after resistance to the main pharmacological treatment for it—chloroquine—arose in the 1950s and spread throughout the world. The disease quickly regained its footing in places where it had been on the verge of elimination and with greater force, as populations had lost their previous immunity. “Mortality rates from malaria skyrocketed,” says Dr. Fidock. The first international effort to eliminate malaria was officially abandoned in 1972.
For decades afterward, no one knew how Plasmodium falciparum, the parasite that causes the most virulent and potentially lethal form of malaria, had acquired the ability to withstand drug treatment. Then, in 1999, Dr. Fidock, working at the NIH with Thomas Wellems, PhD, MD, identified the precise genetic mutations that enabled the parasite to evade the toxic effects of chloroquine. The finding was a landmark discovery in the world of malaria research and helped Dr. Fidock obtain several grants as a new investigator, including an Investigator in Pathogenesis of Infectious Disease Award and a New Initiatives in Malaria Research Award from the Burroughs Wellcome Fund, a Global Infectious Disease Scholar Award from the Ellison Medical Foundation, and a Speaker’s Fund in Biomedical Research Award from the New York Academy of Medicine.
“Tom [Wellems] had applied a classical genetic approach, using a genetic cross in a chimpanzee to localize the genomic region associated with the inheritance of chloroquine resistance,” Dr. Fidock recalls. “That ultimately led to us identifying and describing the gene now known as pfcrt, which we have since found also plays a key role in resistance to many therapies that have since been adopted to replace chloroquine.” That discovery would change the course of malaria research and may yet prove to be the key to ultimately breaking the back of a disease that kills more than 600,000 young children every year, according to global statistics.

The Initiative in Infection, Immunity, and Inflammation, known more simply as “I-4,” aims to gather those previously disparate fields of research under one umbrella to help scientists identify areas of intersection. Harnessing their interconnectedness will enable researchers to work together to develop novel approaches and solutions to diseases that continue to plague the world.
David Fidock’s work in malaria fits into that goal and develops Columbia’s overall strength in these areas of research, says Sankar Ghosh, PhD, the Silverstein and Hutt Family Professor of Microbiology & Immunology, department chair, and leader of I-4. Infection remains a major medical problem in all parts of the world. Pandemics, including malaria, are complicated by drug-resistant pathogens, requiring nuanced public health solutions based on advanced understanding of the molecular and genetic mechanisms that allow these organisms to continue to thwart eradication efforts.
“A malaria vaccine has been a holy grail. The disease is so endemic in poorer parts of the world, a liver-stage vaccine is the only thing that will ultimately make a difference in eradication,” says Dr. Ghosh. “As we think about where our medical school is heading, investment in these key areas of research will be critical to developing our future strength.”
Gene Shapes Malaria Policy
The fact that chloroquine resistance was due to a change in a single gene rather than multiple genes demonstrated that the parasite was capable of acquiring resistance more quickly than anyone had imagined. Field research by Dr. Fidock and others working in countries where malaria is rampant has shown that mutant PfCRT was much more prevalent and more widespread than had been anticipated.
“While PfCRT is not used as a marker to guide treatment for individuals, it has helped shape malaria policy,” says Dr. Fidock. “This was particularly evident in India where chloroquine continued to be used for years after it had been abandoned in Southeast Asia. Overwhelming evidence for the presence of mutant pfcrt alleles throughout India contributed to the Indian government’s recent decision to stop using chloroquine as first-line therapy and instead adopt an effective antimalarial combination.”
Artemisinin-based combination therapies (ACTs) currently constitute the first line of defense against malaria. These therapies—a combination of a fast-acting drug called artemisinin and a slower-acting antimalarial, such as lumefantrine, amodiaquine, or mefloquine—are recommended by the World Health Organization to treat uncomplicated malaria around the world. The global movement away from chloroquine and toward ACTs is thought to be partially responsible for a nearly 30 percent reduction in child mortality worldwide in the past five years, accounting for as many as 100,000 to 200,000 deaths averted per year.
But evidence of emerging resistance to artemisinin along the Thai-Cambodian border shows that new drugs are desperately needed. A major effort under way in Dr. Fidock’s lab is to delineate the genetic and molecular mechanisms that drive resistance to new potential drugs—and find ways to outmaneuver them.
“ACTs are being heavily pushed as the best way to treat malaria around the world, but there is a real concern about resistance spreading from Southeast Asia,” says UCSF malaria expert Philip Rosenthal, MD, who tests new antimalarial regimens in Uganda and Burkina Faso. “That’s why we need laboratory research—like David’s—to take what we are finding in the field and guide us to new treatments.”
New Drug Development: Avoiding Rapid Resistance
The many transitions the malaria parasite goes through in its lifecycle make it something of a moving target for drugs. Most drugs, like chloroquine, target the blood stage of the disease. It’s this “blood stage” that causes disease—and even death in the host. Within the red blood cells, the parasites are safe from detection by circulating immune cells. They replicate at a furious pace, making eight to 24 copies of themselves every 48 hours and filling the cells to near bursting.
“You can get up to 20 percent of all red blood cells infected with the parasite, which can cause hemolytic anemia, a major cause of death in young children who do not have a sufficiently strong immune response specific to this parasite,” says Dr. Fidock.
The parasites then have to pull themselves out of circulation; otherwise the misshapen cells would be cleared by the spleen. The parasites deposit sticky proteins on the surface of their host cells that cause the cells to accumulate inside small blood vessels in different parts of the body. The accumulation of defective cells in microvessels that feed the brain causes cerebral malaria with its associated seizures and coma, especially in children. In pregnant women, the infected cells attach to vessels present in the placenta, which can cause pregnancy complications, disease, and death.
In Dr. Fidock’s lab, researchers expose these blood-stage parasites to promising antimalarial candidates to see how quickly mutations develop to protect the parasites. Drug candidates come from the public-private partnership Medicines for Malaria Venture and industry-based colleagues at the Novartis Institute for Tropical Diseases, GlaxoSmithKline, and Sanofi.
“Exposure to sub-lethal concentrations of the drug will kill most of the parasites, but if some parasites can survive, we know that the organism is capable of developing relatively rapid resistance to the drug in question,” says Marcus Lee, PhD, an associate research scientist in the Fidock lab. “A particularly ineffective drug may give you resistance fairly easily from a relatively small number of parasites, but for some drugs, it’s hard to evolve resistance in the lab; these are potentially good therapeutic candidates.”
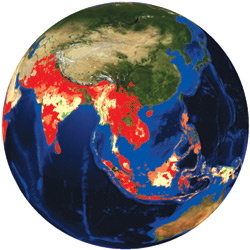 Expanding treatment to more than 90 percent of infected individuals, combined with mosquito control measures, is predicted to reduce transmission of the malaria parasite to such levels that the disease could be eliminated from regions with low to moderate disease transmission. This map shows the predicted probabilities of eliminating malaria if a five-fold reduction in transmission levels is achieved. Probabilities of malaria elimination range from more than 90 percent (bright red) down to less than 50 percent (darkest green). Areas without color indicate no malaria. This calculation, based on a model produced by a team that includes Columbia’s David Fidock, is now being used to help guide malaria elimination efforts.
Expanding treatment to more than 90 percent of infected individuals, combined with mosquito control measures, is predicted to reduce transmission of the malaria parasite to such levels that the disease could be eliminated from regions with low to moderate disease transmission. This map shows the predicted probabilities of eliminating malaria if a five-fold reduction in transmission levels is achieved. Probabilities of malaria elimination range from more than 90 percent (bright red) down to less than 50 percent (darkest green). Areas without color indicate no malaria. This calculation, based on a model produced by a team that includes Columbia’s David Fidock, is now being used to help guide malaria elimination efforts.Even the bad candidates provide useful information for the researchers. If a mutation arises again and again, it is flagged as a potential hotspot of resistance. Identifying the gene that confers resistance does two things: “If we can validate the gene, we can identify the source of the resistance,” says Dr. Lee. “And we can obtain important clues as to the actual cellular target of the drug.” That information can then be parlayed into efforts to identify further potential therapies.
The studies also show how current drugs can be used most effectively, as some genetic mutations that confer resistance to one drug increase susceptibility to another. “PfCRT mutations cause resistance to chloroquine but, curiously, they make parasites more susceptible to mefloquine, one of the drugs used in ACTs,” says Dr. Lee.
Dr. Fidock believes that by leveraging the drug vulnerabilities against one another, they can prevent the parasite from developing genetically stable resistance. Using field data and mathematical models, they can predict the best combinations of drugs to be used in different areas. “In one study, we showed that parasites that are resistant to chloroquine manifested some cross-resistance to amodiaquine, which is combined with artemisinin in a common ACT. But at the same time they were increasingly susceptible to lumefantrine, an alternative combination that is also frequently used,” says Dr. Fidock. In Africa, many countries now routinely use both combinations to exert opposing pressures on the parasite; as parasites become resistant to one combination they become more susceptible to the other.
Reaching for the Holy Grail of Malaria Research
Though the blood stage of the malaria parasite causes the disease, the holy grail of malaria researchers is a drug or vaccine that can stop the parasite at an earlier stage, the liver, preventing not only the disease’s symptoms, but also its further spread. Yet multiple barriers have thwarted the research, from the ability of the parasite to hide from the human immune system to the difficulty in studying the liver stage in the lab.
After leaving an infected mosquito, the parasite makes the liver its first destination in the human host. A relatively small number of sporozoites, the motile life-stage of the parasite, come from a single mosquito bite. Even the small initial inoculum serves the parasite. “There is a survival benefit in the delivery of such a small number of parasites, because they can slip below the immune system’s radar,” says Dr. Fidock.
Within an hour, sporozoites can reach the liver and invade hepatocytes. Once sequestered there, they reproduce for the next six to seven days, with each sporozoite producing up to 30,000 merozoites—which then leave the liver to invade the host’s red blood cells.
During their time in the liver, the sporozoites must either synthesize or scavenge massive amounts of lipids from the host to support the extensive replication that takes place before the symptomatic blood stage. How and where they get hold of those lipids is what Dr. Fidock believes holds the key for the development of a liver stage vaccine.
“If the parasite’s ability to gather or produce lipids could be genetically attenuated, the parasites would arrest in the liver, where they would then persist for weeks and generate a robust immune response from the host,” says Dr. Fidock. “A vaccine loaded with genetically attenuated parasites would deliver far more sporozoites than a mosquito bite, and that would substantially boost the immune response as well.”
Preliminary results from a liver-stage vaccine developed by one of Dr. Fidock’s collaborators suggest that some of the challenges in developing such a vaccine can be met. In a 2013 study published in Science, a vaccine made of irradiated sporozoites provided 100 percent protection to a small number of volunteers. Sanaria, a private biotechnology firm in Maryland, produced the vaccine with funding from the National Institute of Allergy and Infectious Diseases Small Business Innovative Research Program and the Bill & Melinda Gates Foundation.
“This result by Sanaria is ground-breaking because they proved that it is technically possible to produce a cryopreserved malaria vaccine that provides protection against a live sporozoite challenge,” says Dr. Fidock. “Vaccination with irradiated sporozoites had previously been demonstrated but never with sporozoites that had been cryopreserved and stored. This shows us that an effective malaria vaccine can be generated at an industrial scale. The challenge now is how to scale this up to the numbers of doses required to have a significant impact in reducing infant mortality in sub-Saharan Africa.”
By using genetically modified sporozoites in the vaccine, with mutations that specifically target the liver stage, such a vaccine may be easier to produce than one made with irradiation, which hits different genes each time. Sanaria and Dr. Fidock are working together to create such a vaccine.
While a commercially available vaccine is at best years away, the hope is that one day, innovators and researchers like Dr. Fidock will be able to put a stop to the disease that has been racking so many nations in misery and sickness for centuries.
“With what we know about the history of drug resistance in malaria and current early signs of resistance to artemisinins, resources and research to fight malaria are more urgently needed than ever before to eliminate this disease while the opportunity still exists,” says Dr. Fidock. “Anything less than a coordinated, all-out effort by all partners will lead to the continued and unnecessary deaths of hundreds of thousands of children in malaria-endemic areas each year.”
- Log in to post comments


